Chapter Editors: Timothy Fahey and Paul Schaberg
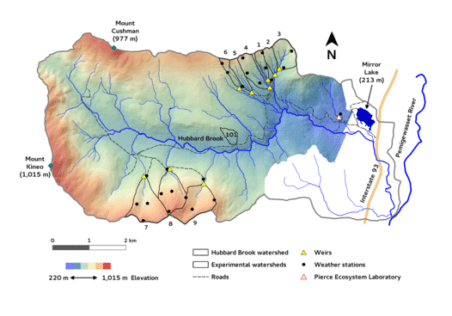
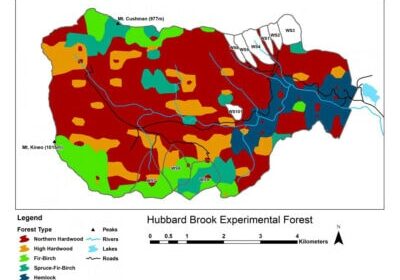
The Hubbard Brook Ecosystem Study was established to use the small watershed approach to measure and model ecosystem element flux and cycling, but it has evolved to incorporate a complex matrix of projects studying a wide range of biogeochemical and ecological phenomenon. Fundamental to understanding many of these phenomenon is information regarding the structure and function of the defining life form there – trees. The northern hardwood-conifer forest that blankets the Hubbard Brook valley is a biological machine that transforms solar energy, water and mineral nutrients into organic matter. It is a complex and beautiful machine arising from millions of years of evolutionary development of anatomical and physiological traits under the influence of the environment and co-existing organisms. What are the key processes that underlie the dynamics of this machine, this forest ecosystem?
How do trees respond to stresses like drought, frost, pollution and to other environmental changes? How does successional change proceed after disturbance events? How much water is diverted from streams by root uptake and transpiration? How efficiently is water used in photosynthetic production? How much of that production is allocated belowground to acquire soil resources? Answers to these and similar questions are all topics that fit under the umbrella of forest physiology - a branch of biology that deals with the structure and function of trees that shape their interactions with and responses to forest environments. At Hubbard Brook, scientists have explored in detail the physiological responses of trees to the stresses associated with acid deposition and consequent changes in soil chemistry as well as altered snowpack that influences soil freezing and root damage. An important subset of physiology that has received increasing interest in light of global change is the study of seasonal phenomena denoted as “phenology” - especially in relation to altered climate norms. Hubbard Brook has one of the world’s most detailed records of tree phenology in spring (budbreak and leaf expansion) and fall (leaf color change and abscission), which have contributed to a better understanding of the drivers and implications of changes in the growing season for deciduous forests. In this chapter we highlight some of the patterns and processes of tree physiology and phenology that have been discovered at Hubbard Brook and in related work in northern hardwood and mixed wood forests.
Understory Tolerance

Figure 1. Sunflecks are transient patches of direct-beam solar radiation associated with small holes in the canopy overstory.
Although it has received only limited study at the HBEF, we begin with a brief review of shade or understory tolerance because this topic is so central to the dynamics of northern hardwood forests that are the foundation for all major ecological phenomenon here. As detailed in Forest Composition and Dynamics chapter, the natural disturbance regime of northeastern U.S. forests is characterized by the death, breakage or uprooting and toppling of individual or small groups of trees, typically during windstorms. This forest disturbance regime has favored tree species that are tolerant of shading by the overstory and can persist in the understory until a canopy gap is created - species like sugar maple, American beech, red spruce, balsam fir and eastern hemlock (Table 1). Although subtle differences exist among these species, they all possess similar key physiological traits, especially regarding their photosynthetic physiology. Light levels beneath a closed northern hardwood canopy are typically less than 1 or 2% of full sunlight. In these conditions leaves must minimize the photosynthetic light compensation point (LCP), the light level at which gross photosynthesis equals leaf respiration and net photosynthesis is zero. Shade tolerant species achieve a low LCP ( <5% of full sunlight) by minimizing leaf respiration per unit of light-absorbing leaf area, which can be accomplished by having thin leaves with only a single layer of chlorophyll-containing mesophyll cells. Conversely, these leaves are unable to capture lots of light under higher illuminations, and hence they exhibit a low light saturation point (the light level above which light no longer limits photosynthesis; e.g., 15% of full sunlight) and low maximum photosynthetic rates. This inevitable trade-off has two key implications for the shade tolerance strategy. First it influences the ability to utilize “sunflecks” (Fig. 1), transient patches of direct-beam solar radiation associated with small holes in the overstory canopy (e.g., due to crown “shyness” when the crowns of fully stocked trees do not touch each other – creating a canopy with channel-like gaps; Fig. 2). Second, when a canopy gap forms as a result of tree fall, the leaves of residual, formerly shaded stock are not well adapted to utilize the consequent high light levels. Physiology_canopy_0.png Figure 2. Crown shyness occurs when the crowns of fully stocked trees do not touch each other, which creates a canopy with channel-like gaps.

Figure 2. Crown shyness occurs when the crowns of fully stocked trees do not touch each other, which creates a canopy with channel-like gaps.
Under a closed canopy in northern hardwood forests, about two-thirds of the total growing season sunlight reaching the understory comes in the form of sunflecks (Way and Pearcy 2012). Thus, it is essential for shade tolerant species to make good use of this radiation. The typical sunfleck lasts only about 4-8 minutes as the sun passes over a hole in the canopy. The average intensity of sunlight during the sunfleck is about one-third of full, direct-beam sunlight, which is still far above the typical photosaturation point of shade leaves. Moreover, under deep shade, most leaves will have stomata closed to conserve water. Thus it might seem advantageous for understory leaves to rapidly open stomata when a sunfleck occurs to allow for the absorption of carbon dioxide (CO2) for photosynthesis. However, in the shade, internal stores of CO2 within leaf mesophyll cells can typically supply the initial pulse of gross photosynthesis without requiring additional CO2 diffusion through stomata. More important is the capacity for rapid activation of enzymes that catalyze the light reactions of photosynthesis. Because of this, shade tolerant leaves are able to briefly store energy from the light reactions to continue powering dark reactions after sunflecks have passed (Way and Pearcy 2012). Vertical (upper) and horizontal (lower) extension growth of sugar maple and beech sapling of three height classes in three light environments (from Poulson and Platt 1996).
| Very Tolerant | Mid-tolerant | Intolerant | Very Intolerant |
|---|---|---|---|
| Hemlock | White pine | Red pine | Larch |
| Balsam fir | Basswood | Black cherry | Pin cherry |
| Red spruce | Red maple | Tulip poplar | Paper birch |
| Black spruce | Yellow birch | Butternut | Heart-leaf birch |
| Beech | Red oak | Choke cherry | Gray birch |
| Sugar maple | White ash | Bigtooth aspen | |
| Striped maple | Quaking aspen | ||
| Hop hornbeam |
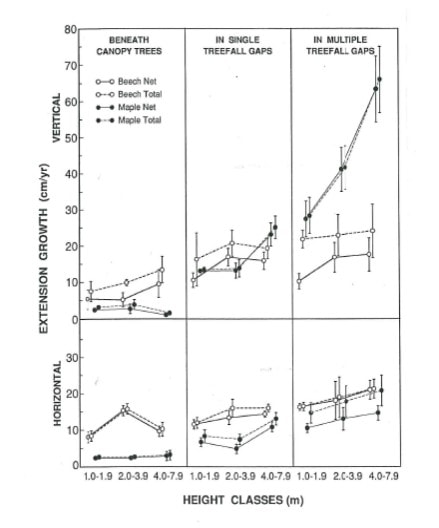
Figure 3. Vertical (upper) and horizontal (lower) extension growth of sugar maple and beech sapling of three height classes in three light environments (from Poulson and Platt 1996).
The ability of tree seedlings to respond with higher growth when a canopy gap forms depends upon changes in leaf and canopy morphology. For highly tolerant, deciduous species like sugar maple and beech, new leaves that are produced by seedlings and saplings in gaps can adjust key traits like leaf thickness and the angle of leaf inclination that will improve a plant’s photosynthetic performance in high-light environments. For evergreen conifers like spruce, fir and hemlock that retain their leaves for many seasons, most foliage must remain shade-adapted for years following gap formation; thus, growth of these species is less responsive to changes in light environments. The important role of canopy architecture in understory adaptation was shown for beech and sugar maple by Canham (1988): beech grows better in deep shade, in part because of the efficient way that it presents its leaves to the sky, whereas maple responds more than beech to gap formation, in part because of its great plasticity in changing branch angles (see Fig. 3). Thus, the coexistence of these tolerant species may be favored by subtle niche separations related to gap dynamics. At another extreme, hemlock shows little growth response to gaps, but is able to survive and grow very slowly during its more extended time in deep shade. Further study of the physiology of coexistence among extremely shade-tolerant species (Table 1) within mixed northern hardwood-conifer forests is needed, especially in light of global change that may increase the frequency and severity of extreme weather events that generate canopy gaps or induce stand replacement (Janowiak et al. 2018).
In the deciduous forest, light availability also fluctuates seasonally as a function of spring leaf out and fall senescence. At Hubbard Brook, Gill et al. (1998) observed that the transmission of photosynthetically active-radiation near the ground surface in hardwood forests declined rapidly from 55% in early May (pre-leaf out) to 5% at the end of May before gradually declining to a midsummer minimum of 1.5-2% in late June. Conversely, light transmission increased with autumn leaf senescence beginning in late September, reaching 37% by late October. Thus, Gill et al. (1998) distinguished four seasons from the standpoint of advanced regeneration of trees: winter (cold), spring light, summer shade, and autumn light. As detailed below, the timing of these functional seasons is rapidly changing with climate warming.
Understory tree seedlings and saplings leaf out earlier and senesce their leaves later than do overstory trees in northern hardwood forests, presumably as an adaptation to take advantage of seasonally higher light levels and increase overall carbon (C) assimilation. Gill et al. (1998) measured photosynthetic activity of tree seedlings in the understory environment and showed that they assimilated significantly more C in the spring light than in the autumn light phase.
The understory environment is resource-limited, not only for light, but also for soil water and mineral nutrients. Overstory trees usually are competing intensively for access to soil resources, often leaving scant supplies for smaller plants. As shown by numerous soil “trenching” experiments, in which roots of dominant trees are excluded from a small plot to reduce competition, growth of tree seedlings and saplings can be limited by soil resources; seedlings grow much faster within trenched plots. The lower the water availability or fertility of soils, the more likely that trenching results in increased plant growth. This response can reflect the relief of acute water or nutrient limitations on photosynthesis and growth, or a relative decrease in C allocation to roots and mycorrhizae that leaves more C for above ground growth. Few trenching studies have been conducted at Hubbard Brook. In one example, Cleavitt et al. (2008a) found that growth of beech seedlings benefitted more than beech sprouts from trenching as would be expected because sprouts are nurtured by parent tree roots.
Leaf conductance, transpiration and forest hydrology
The earliest research on tree physiology at Hubbard Brook was designed to address questions about forest hydrology that were originally the focus of the USFS research program. In particular, could differences in leaf conductance (or resistance) to water vapor diffusion (i.e., loss) among tree species contribute significantly to the water balance of forested watersheds? Federer (1977) measured leaf resistance of dominant trees and shrubs as well as their responses to drought. These values were incorporated into a simulation model of forest water yield (BROOK; Federer and Lash 1978) to show that annual water yield could vary by 15-120 mm as a result of differing species composition. Similarly, Federer (1973) demonstrated that forest transpiration greatly increases the rate of recession (decline) of stream flow during rain-free intervals of the growing season. Gee and Federer (1973) observed that in autumn, transpiration continues unabated as long as leaves remain green, but declines to low values with foliar senescence as indicated by color change.
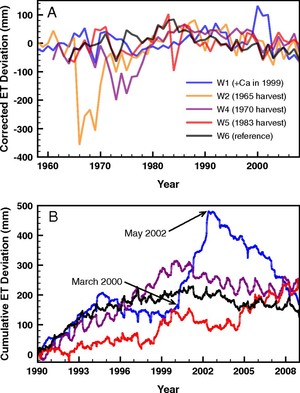
Figure 4. Evapotranspiration deviation from the hydrologic reference (W3) at an (A) annual scale and (B) cumulative daily deviation between 1990 and 2008. Note the striking increase in deviation for W1 from 2000-2003 after Ca addition in 1999 (from Green et al. 2013).
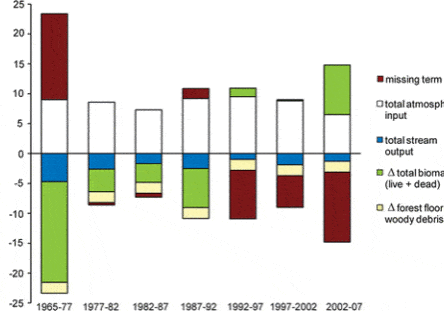
More recently, studies of forest transpiration at Hubbard Brook and the Bartlett Experimental Forest (E.F.) have contributed to a better understanding of hydrologic responses to global change. For example, Hornbeck et al. (1997) demonstrated that the higher leaf conductance of early successional species (birches and cherry) than mature forest dominant species (beech, maples) likely explains the lower water yield of early successional forest watersheds. Considering this, it seems likely that changes in forest composition could interact with climate change to influence hydrologic regimes as well as C exchange. Moreover, increasing concentrations of CO2 in the atmosphere would be expected to result in higher forest water-use efficiency (WUE – a measure of C uptake relative to water loss through stomata) because elevated CO2 can reduce stomatal aperture. In accordance with this, and based in part by measurements from the eddy flux tower at the Bartlett E.F., Keenan et al. (2013) found evidence for gradually increasing WUE in northeastern forests. This apparent pattern may be reflected in the significant long-term declines in annual actual transpiration (AET) from reference watersheds at Hubbard Brook, which have occurred despite warming temperatures and lengthened growing seasons (see Climate Change chapter). Also, the possible effect on forest WUE of sub-canopy water recycling (Green et al. 2015), in which transpired water re-condenses and is used by understory vegetation, deserves further evaluation.
An unexpected result from a calcium (Ca) addition experiment on Watershed 1 (W1) at HBEF was a 20% decrease in streamflow that persisted for three years following treatment (Green et al. 2013; Fig. 4). Subsequent unpublished measurements strongly suggest that increased sap flow and transpiration by mature trees caused this striking hydrologic response, but the mechanism causing this behavior has yet to be demonstrated. Could the de-acidification of northeastern forest soils as inputs of acid deposition decline be altering hydrologic regimes?
Acid deposition and forest physiology
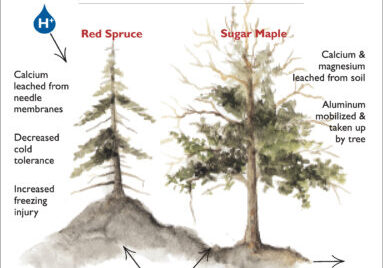
Much of the forest physiology research at Hubbard Brook has resulted from studies to document and understand the decline of two prominent tree species within northeastern forests - red spruce and sugar maple. Early research on these declines was focused first on demonstrating that growth declines and mortality of these species were, in fact, out of the ordinary, and in narrowing down the wide range of possible explanations for these declines (e.g., changes in climate, pollution, pests and pathogens, etc.). During the 1980’s physiological studies in the National Acid Precipitation Assessment Program suggested that, for both species, acid deposition and the depletion of the essential nutrient cation Ca was likely involved. In the case of red spruce, symptoms of foliar winter injury were associated with reductions in cold tolerance caused by the depletion of membrane-associated Ca in foliage (DeHayes et al. 1999). Although the decline of sugar maple appeared to be more complex, again a role for Ca depletion was suggested (Schaberg et al. 2001). Two experiments at Hubbard Brook have contributed to a better understanding of the physiological bases of spruce and maple declines. First, in 1999 W1 was treated with a Ca silicate mineral, wollastonite, in an amount designed to replace the Ca that was lost from the ecosystem during the 20th century (i.e., a restoration of Ca rather than a fertilization treatment). Second, in 1995 plot-level treatments with Ca or aluminum (Al) were initiated in high-elevation, maple-dominated plots (the nutrient perturbation (NuPert) study). The Al treatment was designed to evaluate the independent role of this toxic element (that increases in soils as a result of acidification) in maple decline.
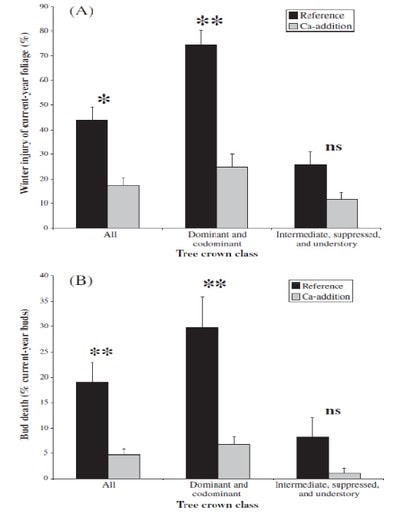
Figure 5. Red spruce winter injury on reference (W6) and Ca addition (W1) watersheds following a severe winter (2003) (A) foliar injury and (B) bud mortality in three crown classes (from Hawley et al. 2006).
Experimental studies in the laboratory on red spruce saplings had illustrated previously that acid deposition can directly leach membrane Ca from foliage thereby reducing foliar cold tolerance and predisposing needles to freeze-induced mortality, which was a principal cause of this species’ decline (DeHayes et al. 1999). The W1 Ca addition experiment provided key evidence that depletion of soil Ca by acid deposition caused mature red spruce trees to be susceptible to freezing injury: the restoration of soil Ca on W1 reduced foliar winter injury well over 50% in comparison to spruce on the adjacent reference watershed following a cold snap in 2003 (Fig. 5; Hawley et al. 2006). Moreover, subsequent measurements on W1 (Halman et al. 2008) and elsewhere (Boyce et al. 2013) demonstrated that elevated foliar sugar concentrations and ascorbate peroxidase activity were associated with higher foliar Ca and needle cold tolerance of spruce following soil Ca restoration. In addition, putative stress protection compounds, including polyamines and free amino acids, increased in spruce foliage in Ca-treated sites, especially during winter, suggesting that higher Ca increased foliar protection and reduced the vulnerability of spruce needles to winter freezing injury (Schaberg et al. 2011).
Declines of sugar maple across its range in eastern North America have been observed periodically for decades (Houston 1999) and attributed to numerous stresses (Westing 1966), including the poor mineral nutrition of trees (Mader and Thompson 1969). It is likely that the etiology of maple decline varies among sites, but a common feature of many maple decline episodes is inadequate Ca nutrition. The reasons why sugar maple trees are highly sensitive to Ca deficiency are not fully understood, but studies at Hubbard Brook have helped to clarify the mechanisms and demonstrate the role of acid deposition in maple decline. Several experiments had previously demonstrated that liming soils can correct maple decline (e.g., Long et al. 1997). The restoration of soil Ca on WS1 at HBEF demonstrated conclusively that depletion of soil Ca by acid deposition was involved in sugar maple decline (Juice et al. 2006): crown condition of mature maples was improved, growth of seedlings was greatly increased, and mycorrhizal colonization of both seedlings and mature trees was strongly enhanced. Restricted mycorrhizal associations in Ca depleted soils were associated with higher tree fine root biomass levels (Fahey et al. 2016), possibly as a compensation to increase Ca uptake when mycorrhizal levels were low. Because higher tree C allocation to roots (Fahey et al. 2016) was associated with depressed aboveground biomass (Battles et al. 2014), it seems likely that a generalized shift in C source-sink relations to favor belowground stores was one mechanism responsible for aboveground declines in sugar maples with Ca depletion.
Additional research on W1 and in the NuPert plots has demonstrated several other physiological features associated with sugar maple decline and recovery. In general, Ca-treated trees used their C resources for growth and reproduction whereas under increased soil Al stress trees devoted more C to defense-based processes. The higher soil Al in NuPert plots caused root damage as indicated by reduced membrane integrity and also increased foliar antioxidant activity (Halman et al. 2013), indicating a role of Al toxicity in maple decline. Moreover, following canopy damage by the 1998 ice storm, maple trees on Ca-treated plots exhibited more rapid wound closure and greater growth release of intermediate and co-dominant trees following crown damage to dominant trees (Huggett et al. 2008). Metabolic indicators of foliar stress in sugar maple were lower on Ca-treated than reference sites (Minocha et al. 2010), especially at higher elevations where soil Ca depletion was most severe. Importantly, maple reproduction (flowering, seed quantity and germination; Halman et al. 2013) and seedling survival (Juice et al. 2006) were significantly improved with Ca restoration. Indeed, Cleavitt et al. (2018) showed that sugar maple has been nearly eliminated from higher elevations of W5 thirty years after a whole-tree harvest, despite the fact that it was the most abundant species in the pre-harvest forest and initially dominated the regenerating forest on W5. They proposed that the severe depletion of soil Ca at high elevations on W5 has disrupted sugar maple physiology, with possible implications for intensive harvests of sugar maples on acidified soils elsewhere and for the future expansion of the species to higher elevations in a warming climate (Cleavitt et al. 2018).
Soil freezing and tree physiology
Science at HBEF has helped to highlight one paradox of a warming climate – that it can increase the risk of freezing injury for at least some plant tissues (Groffman et al. 2012). The accumulation of a deep snowpack early in the winter in the White Mountains normally insulates the soil from frigid temperatures and prevents extensive soil frost from forming (Pierce et al 1958). However, if snowpack development is delayed or midwinter snowmelt occurs, then cold temperatures can cause deep freezing within soils, an occurrence which may be more frequent in our changing climate (Campbell et al. 2005). A series of snow manipulation studies have been conducted at Hubbard Brook to investigate the mechanisms and implications of soil freezing effects on northern hardwood forest ecosystems (e.g., Groffman et al. 2001, 2011; Campbell et al. 2014). From a biogeochemical standpoint, among the key responses to severe soil freezing are an increase in nitrate leaching from soils with consequent pulses of soil acidification and increases in dissolved Al concentrations (Fitzhugh et al. 2001).
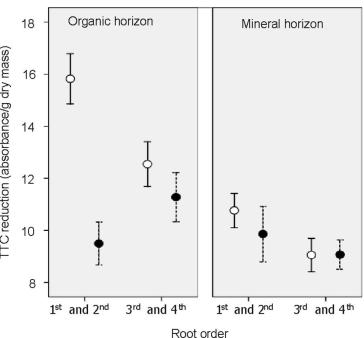
Figure 6. Root cell vitality (TTC reduction assay) of two root orders in organic and mineral soil horizons collected in spring from reference (open circles) and snow removal (soil freezing) plots (from Cleavitt 2008b).
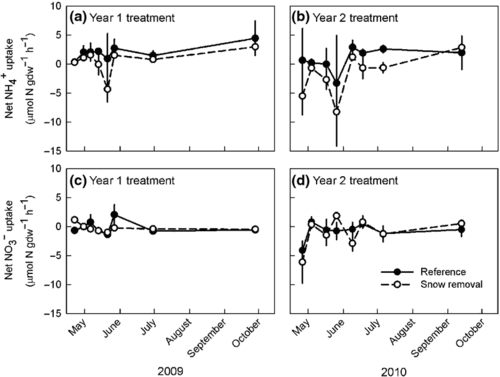
Figure. 7. Uptake of NH4 and NO3 by sugar maple roots in reference and snow removal (soil freezing) plots in two years of treatment (from Campbell et al. 2014).
The interactions between tree physiology and soil freezing effects are complex. Severe soil frost causes extensive sub-lethal damage to roots especially in shallow soil depths as evidenced by decreased root vitality (Fig. 6, Cleavitt et al. 2008b) and increased electrolyte leakage (Comerford et al. 2013); it also may increase root mortality and cause a compensatory increase in root growth early the following growing season (Tierney et al. 2001). Fine root injury results in decreased early-season nitrogen (N) uptake the following summer (Fig. 7), which is the principal cause of increased leaching of nitrate (Campbell et al. 2014). Notably, Campbell et al. (2014) observed rapid N uptake by roots of sugar maple even while the trees were leafless in the spring and fall (Fig. 7). Thus, nutrient uptake by deciduous tree roots can be decoupled from transpiration and photosynthesis, a fact that still needs to be incorporated into coupled models of global climate dynamics. Furthermore, the soil acidification associated with increased nitrate leaching mobilizes soil Al, resulting in stress to sensitive tree species like sugar maple, analogous to that noted earlier for acid deposition. Comerford et al. (2013) observed that soil freezing created cation imbalances that were associated with reduced sugar maple shoot growth that likely resulted in subsequent increases in foliar starch levels as less C was used for woody growth. Considering this, increased soil freezing due to reduced snowpack in a changing climate could exacerbate historic acid deposition-induced base cation losses, with potentially significant implications for forest health in eastern North America (Comerford et al. 2013).
Forest Phenology and climate change
Phenology is defined as the study of seasonal natural phenomena, especially in relation to climate and plant/animal life. Of course, ongoing rapid changes in climate have important implications for forest phenology, which have stimulated interest in this subject both as evidence of long-term climate change patterns (e.g., Likens 2011) and for understanding responses of forest composition and function (e.g., primary productivity; Keenan et al. 2014). Tree phenology at Hubbard Brook has been measured carefully since 1989 and now provides one of the longest detailed accounts of temperate deciduous forest responses to climate change, contributing to a better understanding of the mechanisms underlying phenological patterns and providing an improved basis for predictions of changing phenology (e.g., Richardson et al. 2006, Keenan and Richardson 2015).
Deciduous leaves: general observations. The most obvious and important phenological patterns in temperate forest are the emergence of deciduous leaves in spring that are then shed and are absent during the cold season (October-May at HBEF). This trait is expressed in species from distantly related plant families, and represents a striking example of convergent evolution (Edwards et al. 2017). The evolutionary origins of winter (or cold) deciduous leaves remains uncertain as three distinct hypotheses have been advanced: 1) that deciduous leaves may have originated in warm, seasonally-dry climates (Axelrod 1966); 2) that they may have first appeared in subarctic latitudes as an adaptation to dark winters at a time in the late Cretaceous when sub-freezing temperatures were not common at these high latitudes (Wolfe 1987), and 3) that the deciduous trait may have originated directly in response to cold temperatures in the Miocene (Edwards et al. 2017). Under the first two of these hypotheses, the deciduous trait was proposed to be pre-adapted for cold temperate climates that became widespread in the early Cenozoic (late Miocene). The third hypothesis was proposed more recently, on the basis of phylogenetic and biogeographic studies of the genus Viburnum. Resolving the origins of the deciduous habit could help guide future studies of the physiological mechanisms and environmental cues that regulate leaf emergence, senescence and loss.
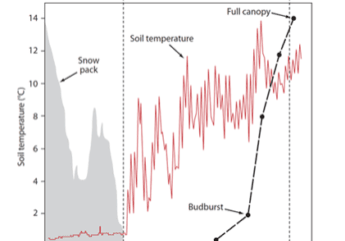
Exactly what environmental cues signal leaf emergence in spring and leaf abscission in fall? The simplest models for spring leaf out utilize a “thermal sum” approach in which accumulated air temperature degree-days (i.e., the sum of °C above a base temperature) are used to predict spring phenological changes (e.g., budbreak, leaf expansion). Although more complex models that incorporate additional environmental drivers, such as day length or a late-winter chilling requirement, may sometimes provide better predictions than the simpler model, they often perform no better (Chuine et al. 1999). Presumably the thermal sum driver is connected physiologically with plant hormone dynamics that coordinate tree responses to changing environmental conditions. For example, rapid increases in soil temperature following the loss of snowpack and subsequent solar warming of dark soils in canopy-less deciduous forests may be an important trigger for instigating root activity that then translates into earlier budbreak and leaf expansion (Groffman et al. 2012). However, as climate change shifts temperatures regimes in complex ways, predictions of spring phenological responses will require more detailed understanding of the physiological processes involved.
The environmental drivers of autumn senescence appear to be more complex than for spring leaf out, and no simple model for this is widely accepted (Schaber and Badeck 2003). Photoperiod, temperature and drought all have been implicated in the breakdown of green chlorophyll pigments that reveal yellow carotenoid pigments already in leaves, which are the most common expression of fall leaf senescence among deciduous trees. However, in some species red anthocyanin pigments can also be newly produced as leaves senesce. A variety of stress agents (e.g., drought, ozone, UV radiation, wounding, etc.) have been shown to trigger anthocyanin synthesis at localized scales (e.g., Murakami et al. 2008), but low temperature exposure is likely the most common trigger of red leaf coloration at the landscape level (Schaberg et al. 2017). Furthermore, it seems likely that differences in the timing and intensity of environmental cues (e.g., moisture limitations, unusual warmth or cold, etc.) could also interact with geographic features (e.g., differences in elevation, aspect, soil depth, features favoring cold air drainage, etc.) to further influence fall color displays over space and time.
Leaf phenology at Hubbard Brook. Nine permanent phenology plots at HBEF are distributed across the valley over an elevational range from 250 to 825m. At the beginning of the study (1989), codominant trees of sugar maple, American beech and yellow birch were permanently marked, and every spring and fall each plot has been visited at roughly weekly intervals through the period of phenological change. For each tree a “phenology index” is recorded, expressing on a scale from 0 (winter condition) to 4 (summer condition) to capture the gradual change in canopy appearance. For example, in spring a rating of 3 corresponds to leaves roughly 50% expanded and in fall a rating of 1 means no more green in the canopy and roughly 50% of leaves have fallen. Richardson et al. (2006) used these measurements to calculate an average phenology index (P) for each species in each year of record, then calculate trends in these over time and relate trends to potential drivers of phenological change. For example, the date on which sugar maple reached a value of P=3 in spring was 2.7±0.4 days later for every 100m increase in elevation; similarly, in fall P=1 was reached 2.5 days earlier for each 100m elevation increase. Hence, canopy duration for sugar maple (interval when canopy is ≥ 50% of full summer) decreases by 5.2 ± 0.6 days per 100m increase in elevation, illustrating the effect of temperature lapse rate on the growing season length (Richardson et al. 2006).
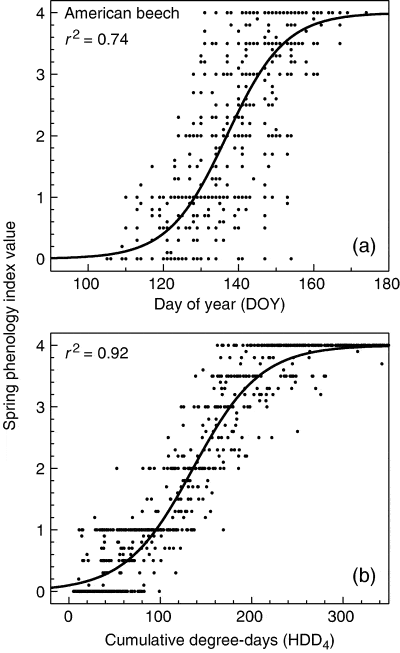
Figure 8 Logistic models of spring phenology index (see text) based on (a) day of year and (b) cumulative heating degree days above 4°C for American beech (from Richardson et al. 2006).
These observations were used to explore predictive models of spring and fall phenology. The most parsimonious model for spring phenology included only a thermal sum above a base temperature of 4°C (Fig. 8), but some interannual variability (17% of residual variance) was not captured by the degree-day model and probably includes other climate effects. Model fits for fall phenology were markedly poorer than for spring and different drivers appeared to give the best fit for the three different species: calendar date for yellow birch and chilling degree-days for maple and beech.
Interestingly, Richardson et al. (2006) compared these phenology models between HBEF and the Harvard Forest and observed that thermal sum models from HBEF overestimated the rate of canopy development at the slightly warmer Harvard Forest. They concluded that such differences in species responses between the sites was probably genetically based. Thus, across the wide geographic range of most species, the development of predictive models could be difficult to produce unless genetic differences are also correlated with climate.
Phenology data from HBEF have been used in combination with satellite observations, ecosystem-scale C exchange measurements and terrestrial biosphere models to evaluate current and future trends in forest C sequestration (Keenan et al. 2014). This analysis suggested that temperature-phenology-C coupling could result in future enhancements of the forest C sink, thereby acting as a negative feedback to climate change, slowing the rate of warming.
Measurements from the HBEF were also combined with retrospective modeling to suggest that spring has been arriving earlier at the rate of about 1.8 days per decade since the 1950s. However, further evaluation of phenological records at HBEF (Keenan and Richardson 2015) indicated that earlier spring leaf out was correlated with earlier fall senescence and vice versa (Fig. 9), so that the duration of the green canopy increased much less than would be expected from an earlier spring alone. Thus, current global models of the response of growing season length in temperate deciduous forests to climate warming probably over-predict future increases in canopy duration, thereby reducing the earlier proposed negative feedback.
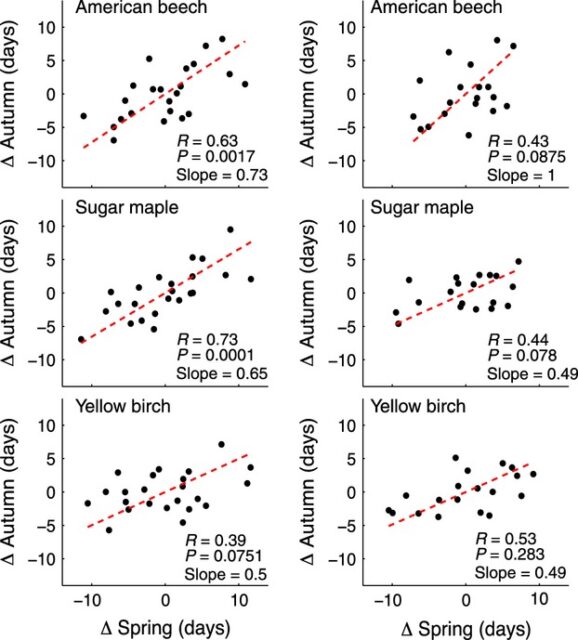
Figure 9. Relationships between spring budburst and autumn senescence for three tree species at Hubbard Brook (left) and Harvard Forest (right), plotted as days of deviation from long term means (from Keenan and Richardson 2015).
An empirical approach to the spring phenology-climate change problem was taken by Friedl et al. (2014) who exploited recent climate anomalies in the Northeast in 2010 and 2012. Specifically, these two springs were by far the warmest on record with temperatures roughly similar to those expected for late in the 21st century (i.e., nearly 3°C warmer than 1971-2000). Ground observations of phenology at HBEF and the Harvard Forest documented leaf out up to two weeks earlier than normal, but with some more complicated thermal forcing factors evident. For example, although spring 2012 was warmer than 2010, leaf out was a week earlier in 2010 (Fig. 10). They concluded that the timing of thermal forcing strongly affected spring phenology, with warm temperatures closer to the time of budburst being much more effective than earlier warm periods. Interestingly, the early leaf out in 2010 was followed by a hard frost that led to significant frost injury to leaves of vulnerable species, including sugar maple on the NuPert plots (Halman et al. 2013). Although Ca treatment there did not seem to influence frost injury, it was associated with a greater re-flush of foliage following injury – thereby providing another indication of the possible interaction of acid deposition-induced changes in physiology with climate-related impacts and responses (Halman et al. 2013).
Continuing development of long-term data sets on the phenology of temperate deciduous trees will likely provide valuable new insights into the implications of climate change for forest dynamics. Coincident measurements of forest physiology and ecosystem C exchange will allow global modelers to more accurately forecast the future of forest C sinks and global climate feedbacks.
Physiology_leaf_emergence.png
Figure 10. Timing of leaf emergence at Harvard Forest (left) and Hubbard Brook (right) for 2010, 2012 and long-term means (all). Width in the plots shows density of observations as a function of day of year (DOY) and blue dots indicate median values (from Friedl et al. 2014).
Questions for Further Study
- Does soil nutrient availability differentially affect the shade tolerance of beech, maple and hemlock and which nutrient is most important to seedling tolerance?
- Is the decline of actual evapotranspiration at Hubbard Brook driven by an increase in tree water-use efficiency in response to increasing atmospheric CO2 concentration?
- What is the mechanism that underlies the striking change in transpiration that followed the addition of calcium to WS1?
- How is ongoing deacidification of soils interacting with climate change drivers to influence physiological processes in northern hardwood trees?
- Why is sugar maple so sensitive to soil calcium depletion and is there natural variation in this sensitivity that could contribute to restoration of this iconic species on acid stressed soils?
- What environmental and internal biotic factors regulate the timing of fall senescence; i.e. what is the internal “clock” that causes the correlation between spring leaf out and fall senescence?
References
Axelrod, D. I. 1966. Origin of deciduous and evergreen habits in temperate forests. Evolution 20(1): 1-15.
Battles, J.J., Fahey, T.J., Driscoll, C.T., Blum, J.D., and Johnson, C.E. 2014. Restoring soil calcium reverses forest decline. Environmental Science and Technology Letters 1(1)15-19.
Boyce, R. L., Schaberg, P. G., Hawley, G. J., Halman, J. M., and Murakami, P. F. 2013. Effects of soil calcium and aluminum on the physiology of balsam fir and red spruce saplings in northern New England. Trees 27(6):1657-1667.
Canham, C. D. 1988. Growth and canopy architecture of shade‐tolerant trees: response to canopy gaps. Ecology 69(3):786-795.
Campbell, John L, Myron J Mitchell, Peter M Groffman, Lynn M Christenson, and J. P Hardy. 2005. Winter in Northeastern North America: a critical period for ecological processes. Frontiers in Ecology 3(6):314-322.
Campbell, J. L., Socci, A. M., and Templer, P. H. 2014. Increased nitrogen leaching following soil freezing is due to decreased root uptake in a northern hardwood forest. Global Change Biology 20(8):2663-2673.
Chuine, I., Cour, P., and Rousseau, D. D. 1999. Selecting models to predict the timing of flowering of temperate trees: implications for tree phenology modelling. Plant, Cell and Environment 22(1):1-13.
Cleavitt, N. L., Fairbairn, M., and Fahey, T. J. 2008a. Growth and survivorship of American beech (Fagus grandifolia Ehrh.) seedlings in a northern hardwood forest following a mast event. The Journal of the Torrey Botanical Society 135(3):328-345.
Cleavitt, N. L., Fahey, T. J., Groffman, P. M., Hardy, J. P., Henry, K. S., and Driscoll, C. T. 2008b. Effects of soil freezing on fine roots in a northern hardwood forest. Canadian Journal of Forest Research 38(1):82-91.
Cleavitt, N.L., Battles, J.J., Johnson, C.E., and Fahey, T.J. 2018. Long-term decline of sugar maple following forest harvest, Hubbard Brook Experimental Forest, New Hampshire. Canadian Journal of Forest Research 48:23-31.
Comerford, D. P., Schaberg, P. G., Templer, P. H., Socci, A. M., Campbell, J. L., and Wallin, K. F. 2013. Influence of experimental snow removal on root and canopy physiology of sugar maple trees in a northern hardwood forest. Oecologia 171(1):261-269.
DeHayes, D. H., Schaberg, P. G., Hawley, G. J., and Strimbeck, G. R. 1999. Acid rain impacts on calcium nutrition and forest health: alteration of membrane-associated calcium leads to membrane destabilization and foliar injury in red spruce. BioScience 49(10):789-800.
Edwards, E. J., Chatelet, D. S., Chen, B-C., Ong, J. Y., Tagane, S., Kanemitsu, H., Tagawa, K., Teramoto, K., Park, B., Chung, K-F., Hu, J-M., Yahara, T., and Donoghue, M.J. 2017. Convergence, consilience, and the evolution of temperate deciduous forests. The American Naturalist 190(S1):S87-S104.
Fahey, T.J., Heinz, A.K., Battles, J.J., Fisk, M.C., Driscoll, C.T., Blum, J.D., and Johnson, C.E. 2016. Fine root biomass declined in response to restoration of soil calcium in a northern hardwood forest. . Canadian Journal of Forest Research 46:738-744.
Federer, C. A. 1973. Forest Transpiration Greatly Speeds Streamflow Recession. Water Resources Research 9(6): 1599-1604.
Federer, C. A. 1977. Leaf resistance and xylem potential differ among broadleaved species. Forest Science 23(4):411-419.
Federer, C. A., and Lash, D. 1978. Simulated streamflow response to possible differences in transpiration among species of hardwood trees. Water Resources Research 14(6):1089-1097.
Fitzhugh, R. D., Driscoll, C. T., Groffman, P. M., Tierney, G. L., Fahey, T. J., and Hardy, J. P. 2001. Effects of soil freezing disturbance on soil solution nitrogen, phosphorus, and carbon chemistry in a northern hardwood ecosystem. Biogeochemistry 56(2):215-238.
Friedl, M. A., Gray, J. M., Melaas, E. K., Richardson, A. D., Hufkens, K., Keenan, T. F., Bailey, A., and O’Keefe, J. 2014. A tale of two springs: using recent climate anomalies to characterize the sensitivity of temperate forest phenology to climate change. Environmental Research Letters 9(5):054006.
Gee, G. W., and Federer, C. A. 1972. Stomatal resistance during senescence of hardwood leaves. Water Resources Research 8(6):1456-1460.
Gill, D. S., Amthor, J. S., and Bormann, F. H. (1998). Leaf phenology, photosynthesis, and the persistence of saplings and shrubs in a mature northern hardwood forest. Tree Physiology 18(5):281-289.
Green, M. B., Bailey, A. S., Bailey, S. W., Battles, J. J., Campbell, J. L., Driscoll, C. T., Fahey, T., Lepine, L., Likens, G., Ollinger, S., and Schaberg, P. G. 2013. Decreased water flowing from a forest amended with calcium silicate. Proceedings of the National Academy of Sciences 110(15):5999-6003.
Green, M.B., B.K. Laursen, J.L. Campbell, K.J. McGuire, and E.P. Kelsey. 2015. Stable Water Isotopes Suggest Sub-Canopy Water Recycling In A Northern Forested Catchment. Hydrological Processes 29(25): 5193 – 5202.
Groffman, P. M., Driscoll, C. T., Fahey, T. J., Hardy, J. P., Fitzhugh, R. D., and Tierney, G. L. 2001. Colder soils in a warmer world: a snow manipulation study in a northern hardwood forest ecosystem. Biogeochemistry 56(2):135-150.
Groffman, P. M., Hardy, J. P., Fashu-Kanu, S., Driscoll, C. T., Cleavitt, N. L., Fahey, T. J., and Fisk, M. C. 2011. Snow depth, soil freezing and nitrogen cycling in a northern hardwood forest landscape. Biogeochemistry 102(1-3):223-238.
Groffman, P.M., Rustad, L.E., Templer, P.H., Campbell, J.L., Christenson, L.M., Lany, N.K., Socci, A.M., Vadeboncouer, M.A., Schaberg, P.G., Wilson, G.F., Driscoll, C.T., Fahey, T.J., Fisk, M.C., Goodale, C.L., Green, M.B., Hamburg, S.P., Johnson, C.E., Mitchell, M.J., Morse, J.L., Pardo, L.H., and Rodenhouse N.L. 2012. Climate change effects are manifest in complex and surprising ways in the northern hardwood forest. BioScience 62:10561066.
Halman, J. M., Schaberg, P. G., Hawley, G. J., and Eagar, C. 2008. Calcium addition at the Hubbard Brook Experimental Forest increases sugar storage, antioxidant activity and cold tolerance in native red spruce (Picea rubens). Tree Physiology 28(6):855-862.
Halman, J. M., Schaberg, P. G., Hawley, G. J., Pardo, L. H., and Fahey, T. J. 2013. Calcium and aluminum impacts on sugar maple physiology in a northern hardwood forest. Tree Physiology 33(11):1242-1251.
Hawley, G. J., Schaberg, P. G., Eagar, C., and Borer, C. H. 2006. Calcium addition at the Hubbard Brook Experimental Forest reduced winter injury to red spruce in a high-injury year. Canadian Journal of Forest Research 36(10):2544-2549.
Hornbeck, J. W., Martin, C. W., and Eagar, C. (1997). Summary of water yield experiments at Hubbard Brook experimental forest, New Hampshire. Canadian Journal of Forest Research 27(12):2043-2052.
Houston, D. R. 1999. History of sugar maple decline. In: Horsley, Stephen B.; Long, Robert P., eds. Sugar maple ecology and health: proceedings of an international symposium; 1998 June 2-4; Warren, PA. Gen. Tech. Rep. NE-261. Radnor, PA: US Department of Agriculture, Forest Service, Northeastern Research Station: 19-26. (Vol. 261).
Huggett, B. A., Schaberg, P. G., Hawley, G. J., and Eagar, C. 2007. Long-term calcium addition increases growth release, wound closure, and health of sugar maple (Acer saccharum) trees at the Hubbard Brook Experimental Forest. Canadian Journal of Forest Research 37(9):1692-1700.
Janowiak, M.K., A. D’Amato, C.W. Swanston, L. Iverson, F. Thompson III, W. Dijak, S. Matthews, M. Peters, A. Prasad, J.S. Fraser, L.A. Brandt, P.R. Butler, S.D. Handler, P.D. Shannon, D. Burbank, J. Campbell, C. Cogbill, M.J. Duveneck, M. Emery, N. Fisichelli, J. Foster, J. Hushaw, L. Kenefic, A. Mahaffey, T.L. Morelli, N. Reo, P. Schaberg, K.R. Simmons, A. Weiskittel, S. Wilmot, D. Hollinger, E. Lane, L. Rustad, P. Templer. 2018. New England Forest ecosystem vulnerability assessment: a report from the New England Climate Change Response Framework. General Technical Report NRS-173. Newtown Square, PA: U.S. Department of Agriculture, Forest Service, Northern Research Station.
Juice, S. M., Fahey, T. J., Siccama, T. G., Driscoll, C. T., Denny, E. G., Eagar, C., Cleavitt, N. L. Minocha, R., and Richardson, A.D. 2006. Response of sugar maple to calcium addition to northern hardwood forest at Hubbard Brook, NH. Ecology 87:1267-1280.
Keenan, T. F., Hollinger, D. Y., Bohrer, G., Dragoni, D., Munger, J. W., Schmid, H. P., and Richardson, A. D. 2013. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 499(7458):324.
Keenan, T. F., Gray, J., Friedl, M. A., Toomey, M., Bohrer, G., Hollinger, D. Y., Munger, J.W., O’Keefe, J., Schmid, H.P., Wing, I.S., Yang, B., and Andrew D. Richardson, A.D. 2014. Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nature Climate Change 4(7):598.
Keenan, T. F., and Richardson, A. D. 2015. The timing of autumn senescence is affected by the timing of spring phenology: implications for predictive models. Global Change Biology 21(7):2634-2641.
Likens, Gene E. 2011. Limnological measures related to climate change in the Hubbard Brook Valley, USA. Inland Waters 1(2):93-99.
Long, R. P., Horsley, S. B., and Lilja, P. R. 1997. Impact of forest liming on growth and crown vigor of sugar maple and associated hardwoods. Canadian Journal of Forest Research 27(10):1560-1573.
Mader, D. L., and Thompson, B. W. 1969. Foliar and Soil Nutrients in Relation to Sugar Maple Decline 1. Soil Science Society of America Journal 33(5):794-800.
Minocha, R., Long, S., Thangavel, P., Minocha, S. C., Eagar, C., and Driscoll, C. T. 2010. Elevation dependent sensitivity of northern hardwoods to Ca addition at Hubbard Brook Experimental Forest, NH, USA. Forest Ecology and Management 260(12):2115-2124.
Murakami, P.F., Schaberg, P.G., and Shane, J.B. 2008. Stem girdling manipulates leaf sugar concentrations and anthocyanin expression in sugar maple trees during autumn. Tree Physiology 28:1467-1473.
Pierce, R. S., Lull, H. W., and Storey, H. C. 1958. Influence of land use and forest condition on soil freezing and snow depth. Forest Science 4(3):246-263.
Poulson, T. L., and Platt, W. J. (1996). Replacement patterns of beech and sugar maple in Warren Woods, Michigan. Ecology 77(4):1234-1253.
Richardson, A. D., Bailey, A. S., Denny, E. G., Martin, C. W., and O’Keefe, J. 2006. Phenology of a northern hardwood forest canopy. Global Change Biology 12(7):1174-1188.
Schaber, J., and Badeck, F. W. (2003). Physiology-based phenology models for forest tree species in Germany. International Journal of Biometeorology 47(4):193-201.
Schaberg, P. G., DeHayes, D. H., and Hawley, G. J. 2001. Anthropogenic calcium depletion: a unique threat to forest ecosystem health? Ecosystem Health 7(4):214-228.
Schaberg, P. G., Minocha, R., Long, S., Halman, J. M., Hawley, G. J., and Eagar, C. 2011. Calcium addition at the Hubbard Brook Experimental Forest increases the capacity for stress tolerance and carbon capture in red spruce (Picea rubens) trees during the cold season. Trees 25(6):1053-1061.
Schaberg, P.G., Murakami, P.F., Butnor, J.R., and Hawley, G.J. 2017. Experimental branch cooling increases foliar sugar and anthocyanin concentrations in sugar maple at the end of the growing season. Canadian Journal of Forest Research 47:696-701.
Tierney, G. L., Fahey, T. J., Groffman, P. M., Hardy, J. P., Fitzhugh, R. D., and Driscoll, C. T. 2001. Soil freezing alters fine root dynamics in a northern hardwood forest. Biogeochemistry 56(2):175-190.
Way, D. A., and Pearcy, R. W. (2012. Sunflecks in trees and forests: from photosynthetic physiology to global change biology. Tree Physiology 32(9):1066-1081.
Westing, A. H. 1966. Sugar maple decline: an evaluation. Economic Botany 20(2):196-212.
Wolfe, J. A. 1987. Late Cretaceous-Cenozoic history of deciduousness and the terminal Cretaceous event. Paleobiology 13(2):215-226.
- Does soil nutrient availability differentially affect the shade tolerance of beech, maple and hemlock and which nutrient is most important to seedling tolerance?
- Is the decline of actual evapotranspiration at Hubbard Brook driven by an increase in tree water-use efficiency in response to increasing atmospheric CO2 concentration?
- What is the mechanism that underlies the striking change in transpiration that followed the addition of calcium to WS1?
- How is ongoing deacidification of soils interacting with climate change drivers to influence physiological processes in northern hardwood trees?
- Why is sugar maple so sensitive to soil calcium depletion and is there natural variation in this sensitivity that could contribute to restoration of this iconic species on acid stressed soils?
- What environmental and internal biotic factors regulate the timing of fall senescence; i.e. what is the internal “clock” that causes the correlation between spring leaf out and fall senescence?